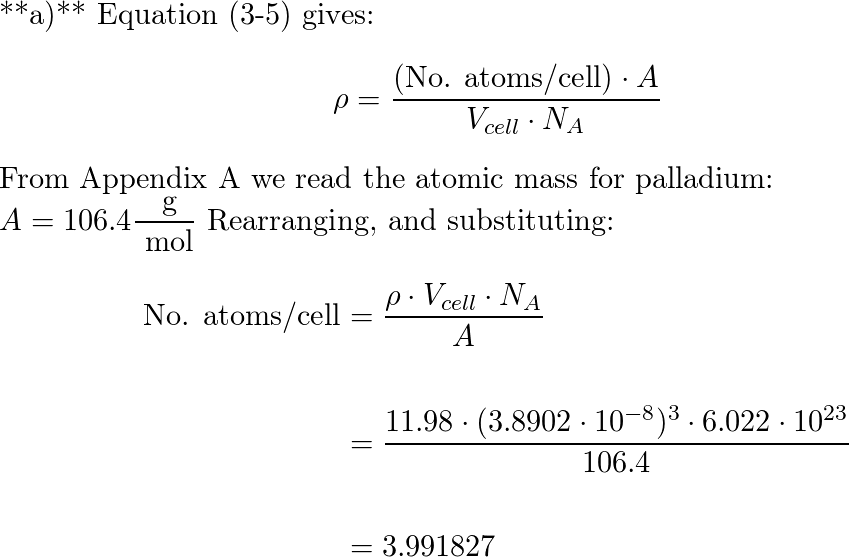HW2 answer 1 - 3.9 Calculate the radius of a palladium Pd atom given that Pd has an FCC crystal structure a density of 12.0 g/cm3 and an atomic | Course Hero

Exam 1 Review Solutions.docx - Exam 1 Review 1. Calculate the radius of a palladium Pd atom given that Pd has an FCC crystal structure a density of | Course Hero
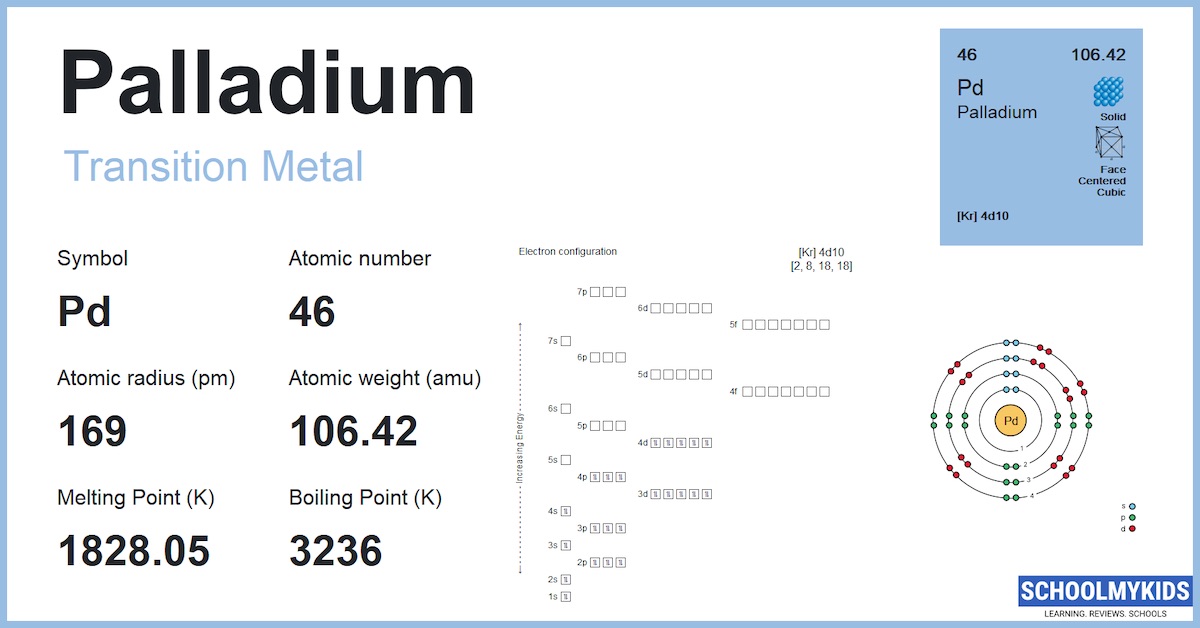
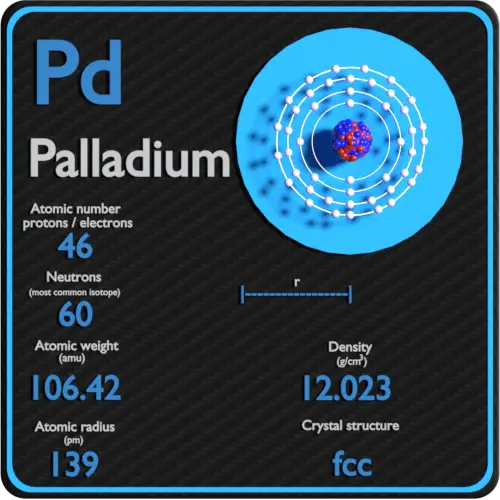
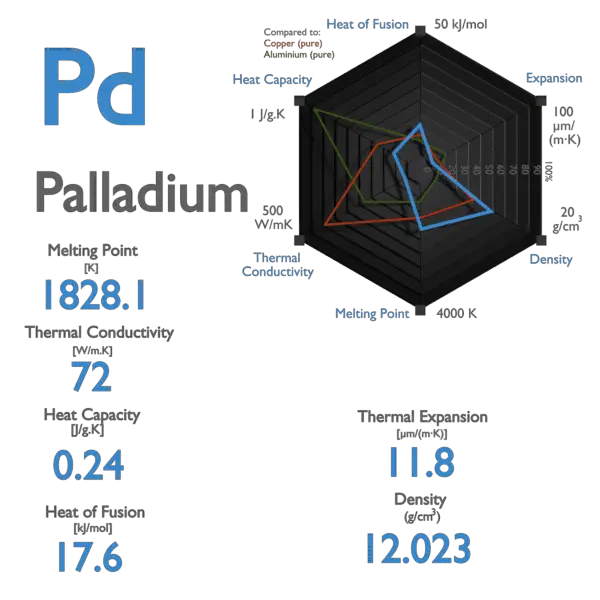
Pd Palladium Element Information: Facts, Properties, Trends, Uses and comparison - Periodic Table of the Elements | SchoolMyKids

the density of palladium is 12.0g/Cm^3. what volume in liters would be occupied by 532 g of palladium? - Brainly.com
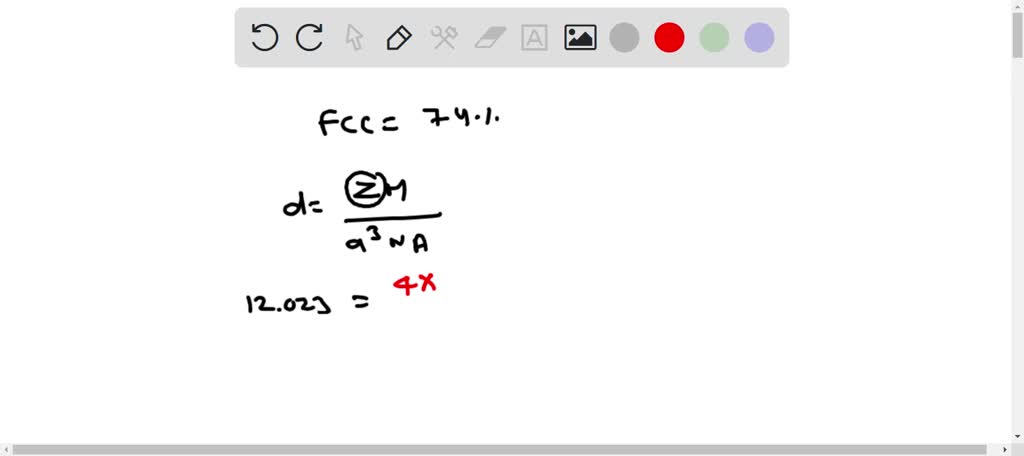
SOLVED: Palladium (at. wt. = 106) crystallizes in a face-centered cubic unit cell. Its density is 12.023 g/cm3 . Calculate the atomic radius of palladium and its packing efficiency.

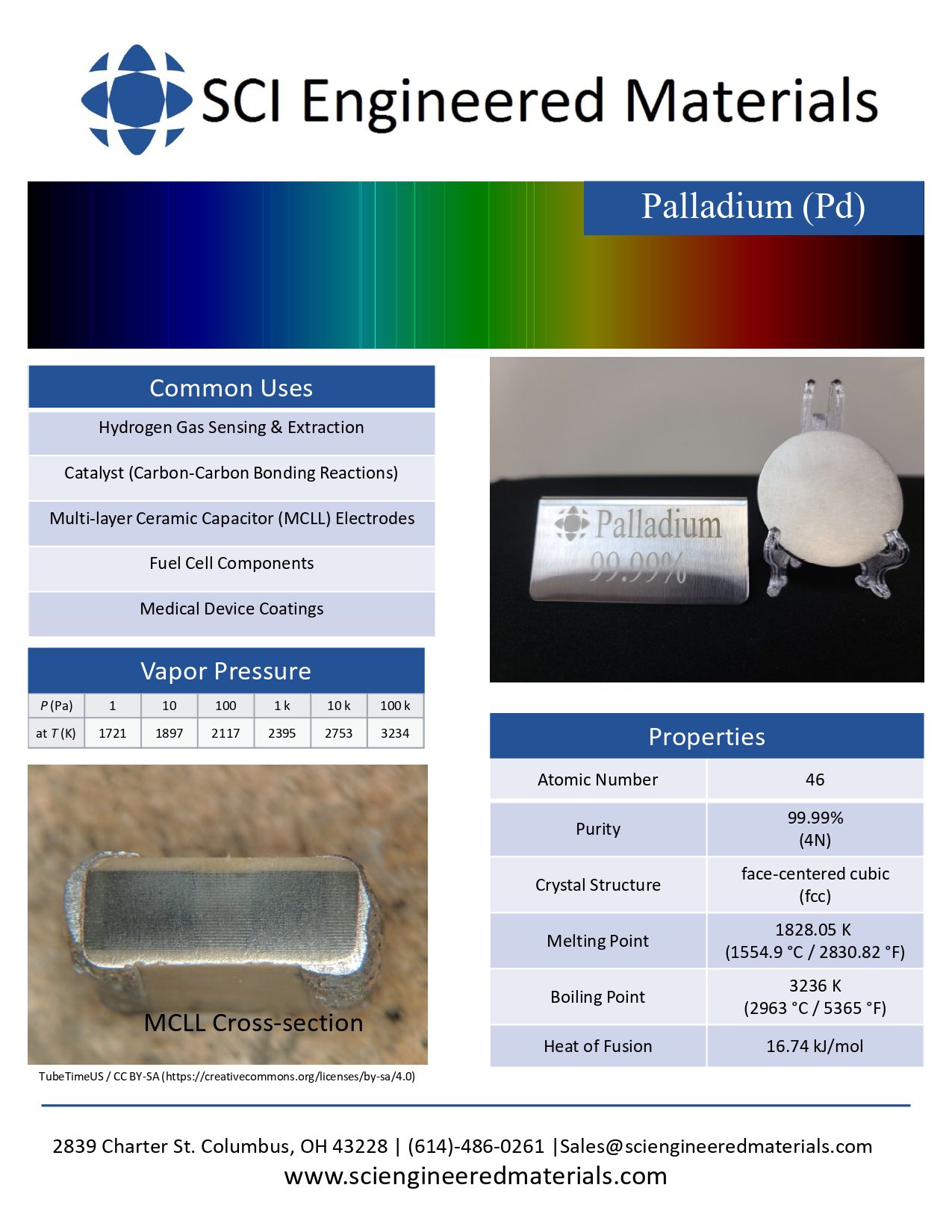
Kurt J. Lesker Company | Palladium Pd Pellets Evaporation Materials | Enabling Technology for a Better World

OneClass: Palladium crystallizes with a face-centered cubic structure. It hasa density of 12.0 g/cm3,...

SOLVED: Calculate the radius of a palladium atom in nm, given that Pd has an FCC crystal structure, a density of 12.0 g/cm3 and an atomic weight of 106.4 g/mol. A. 138